iPV is Insilicom's AI-powered solution to the pharmacovigilance (PV) industry. Currently, we offer an AI-powered drug adverse event literature monitoring system. This tutorial provides a detailed description of the functions it offers.
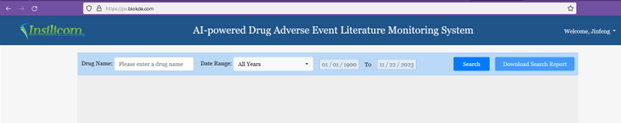
The landing page of iPV (Figure 1) provides inputs for a drug name and a date range to search for adverse events published in PubMed literature during the given date range for the drug.

On the upper right corner of the page, users can click their username to get a dropdown menu, from which they can edit their profiles or contact us through the contact page. The link to the tutorial page of the API tool can be found at the bottom of the page.
Please note that free users can only search for adverse events for limited number of designated drugs. To designate the drugs, please go to the Profile page, and click the Drug Store Settings tab.
We will use Aspirin as an example. Inputting Aspirin, selecting last 1 year, and clicking the Search button will give us the output shown in Figure 2.
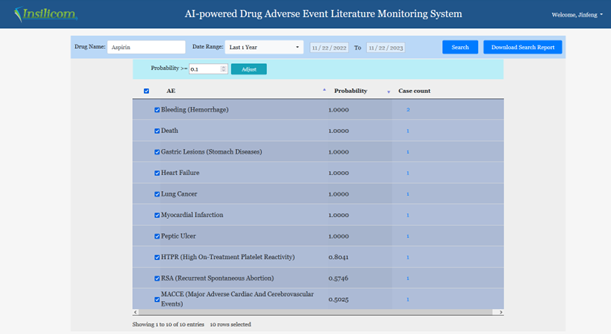
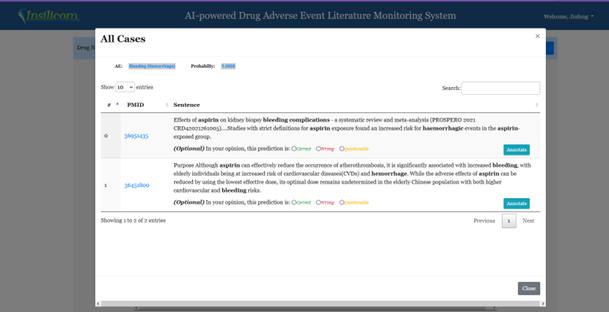
The probability threshold window can be used to filter out cases with lower probabilities. The selection boxes can also be used to select or deselect any specific results. The third column is the number of cases published in the literature during the chosen time frame. Clicking the numbers will bring up a window shown in Figure 3. This window contains the actual description of the adverse events in the published literature. Users can annotate the results by clicking one of the three radio buttons. Alternatively, comments can be added by clicking the Annotate button. Those cases that are annotated as wrong will not be included in the downloaded file.
Users can download the result using the Download Search Report button. The downloaded file contains information on Drug name, AE, probability, PMID, URL, and the sentences describing the adverse events (Figure 4).